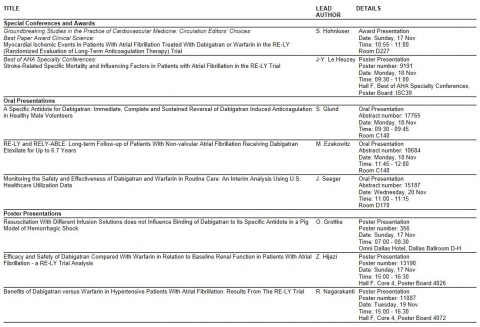
New efficacy and safety data for Pradaxa® (dabigatran etexilate) to be announced at American Heart Association’s Scientific Sessions 2013
•Pradaxa® is the only novel oral anticoagulant with long term clinical evidence – over six years of patient follow up data to be presented1
•Key published Pradaxa® data to receive ‘Best Paper in Clinical Science Award’ and feature in ‘Best of AHA Specialty Conference’2,3
•First results from healthy volunteer study of antidote for reversal of dabigatran-induced anticoagulation4
Boehringer Ingelheim today announces the upcoming presentation of the latest efficacy and safety data for the novel oral anticoagulant Pradaxa® (dabigatran etexilate) at the 2013 American Heart Association’s (AHA) Scientific Sessions, 16-20 November 2013 in Dallas, USA. Data from eight presentations sponsored by Boehringer Ingelheim are included in the Scientific Programme, adding to the growing body of evidence on the clinical benefits of Pradaxa®.
Presentations will include the first results from a healthy volunteer study of a dabigatran-specific antidote as an additional option for physicians in emergency situations where the reversal of the anticoagulation effect of Pradaxa® is required.4 In addition, unique outcome data from over six years of follow-up data from the pivotal Phase III RE-LY® trial and its extension study RELY-ABLE® will be announced,1 as well as real-world data from routine care on Pradaxa® compared to warfarin in patients with non-valvular atrial fibrillation.5 This data will provide further insights into the safety and efficacy of Pradaxa®.
“The American Heart Association’s Scientific Sessions provide a vital forum for physicians to share the most recent clinical developments in the field of cardiology”, said Professor Klaus Dugi, Corporate Senior Vice President Medicine, Boehringer Ingelheim. “At Boehringer Ingelheim, we are committed to driving research and innovation forward in anticoagulation. We are thus very proud to be able to present unique long-term data on Pradaxa® at this congress, as well as the first clinical results from our own development programme of a specific antidote for dabigatran.”
In addition, previously presented clinical research on Pradaxa® from the RE-LY® trial, one of the largest clinical studies ever conducted in stroke prevention in patients with non-valvular atrial fibrillation, will be recognised and awarded during the congress, highlighting the scientific value the work around this trial continues to provide to the medical community. An article by Prof. Stefan Hohnloser, University Hospital, Frankfurt, Germany, which was originally published in 2012, has been chosen by the Circulation editors to receive the ‘Best Paper Award: Clinical Science’. The article analysed data on myocardial ischaemic events reported in the RE-LY® trial and concluded that for the broader composite endpoint of relevant cardiovascular ischaemic eventsi, the incidence was numerically lower with dabigatran than with warfarin.2 A scientific presentation by Prof. Jean-Yves Le Heuzey, European Georges Pompidou Hospital, Paris, France, first shown at the International Stroke Conference 2013, will be featured in the ‘Best of AHA Specialty Conferences’.3
An overview of the Pradaxa® abstracts being presented during the American Heart Association’s Scientific Sessions are listed below. Further information on the Scientific Programme is available at: http://www.abstractsonline.com/plan/start.aspx?mkey=%7b951E351E-429C-4B2E-84D0-8DA73B00DE45%7d
Pradaxa® is currently approved in over 100 countries worldwide for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation and for the primary prevention of VTE following total hip replacement or total knee replacement surgery.6 The extensive in-market experience of over 2 million patient-years puts Pradaxa® first among the novel oral anticoagulants.6
i Composite endpoint including myocardial infarction, unstable angina, cardiac arrest and cardiac death
~ENDS~
Please click on the link for ‘Notes to Editors’ and ‘References’:
http://www.boehringer-ingelheim.com/news/news_releases/press_releases/2013/12_november_2013_dabigatranetexilate.html
Website: http://www.boehringer-ingelheim.com
Contact
Sara McClelland
Phone: +49 6132 – 77 8271
Fax: +49 6132 – 77 6601
Email: press@boehringer-ingelheim.com
This news is a press release provided by Boehringer Ingelheim International GmbH.