Votem Co., Ltd. will participate at MEDICA 2012 to spur Korean Wave for medical equipment
MEDICA 2012, which allows participants to grasp at a glance the latest trends of medical equipment and the new technologies, is regarded as the largest medical equipment exhibition. It is expected that this exhibition will feature approximately 4,000 companies from more than 80 countries.
Votem Co., Ltd. is slated to exhibit its new products VM-150 and VP-700 SNT at this exhibition. VM-15 was designed to allow customers to choose and install one from the monitors of 12.1” TFT LCD (800*600) and 15” TFT LCD (1024*768).
It enhanced the user experience since it is possible to install various monitors additionally such as 19”, 21”, 24”, 27”, etc. through the external VGA and it can be installed in a separate place.
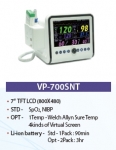
The jointly released VP-700 SNT has the unique feature of being composed of several cassettes in order to install the module, which has ECG, SpO2, NIBP, Resp and 1Temp as the basic elements and other various functions. It also allows customers to use it with much upgraded function if purchasing and installing only the desired module cassette in the future when using the product.
It does not need ECG module, and it is the product that was developed to satisfy the customers’ needs who only wants to use the functions of SpO2 and NIBP; thus, it has significantly improved the functionality of VP-700 that was already verified by the users and it added the module option to measure the body temperature of patients rapidly through installing the Sure Temp of Welch Allyn Co., Ltd.
In addition, it took the advantages of the existing products of Votem Co., Ltd. It was designed to easily upgrade all the modules installed in this product only with SD card without being disassembled. Thus, there is very little of the cost of failure (F-cost) due to user's complaints and upgrades.
“We plan to promote aggressively our new products such as VM-15, VP-700, etc. through participating at ‘MEDICA 2012’ this year. We will leverage this as the starting point to become a ‘global medical equipment specializing company’ through introducing a variety of our patient-monitoring devices at the exhibition,” CEO Tony Kang said.
Contact
Votem Co., Ltd.
+82-33-910-0701
Send Email
This news is a press release provided by VOTEM.