Promising disease modifying approach to Duchenne muscular dystrophy with Neu-REFIX® Beta 1,3-1,6 glucan* from Japan; the first such clinical report
Safety, Fibrosis control and increased muscle strength reported clinically
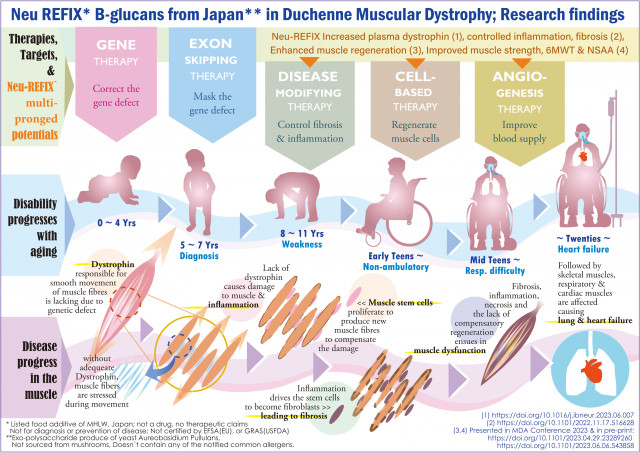
Duchenne Muscular Dystrophy (DMD); Progress of disease & gradual disability with aging, current therapies & Neu-REFIX Beta glucans' multipronged potentials, illustrated. A rare genetic disease with approximately 5000 patients in Japan, 3000 in GCC, fewer than 50000 in USA. Lack of dystrophin in DMD causes muscle dysfunction and makes the patient wheelchair bound in early teens. Lung function deterioration followed by myocardial fibrosis and heart failure causing early death in 20s ~ 30s. Gene therapy targets gene defect correction, exon skipping therapies mask the defect of specific exons. Safety proven Neu-REFIX Beta glucans from Japan, a food additive with multipronged potentials: (i) controlled muscle damage, (ii) enhanced muscle regeneration (iii) improved blood supply marker: plasma dystrophin & (iv) improved 6MWT & NSAA, yielding hope of delaying the disease progress, worth further validation; is not a drug or remedy; Not GRAS or EFSA certified. Approval status varies country wise. (Graphic: Business Wire)
Neu-REFIX reduced fibrosis, inflammation, enhanced muscle regeneration, in MDX mice and in a six-month-long clinical study in young boys, improved NSAA, 6MWT and MRC, and these disease modifying potentials were presented in MDA Conference 2023. Neu-REFIX, can be consumed at any stage of DMD, with standard of care medications. Therefore, it’s worth evaluating as a drug-adjuvant because, as reported, Neu-REFIX as a single agent, has helped accomplish, what the current disease modifying treatments, cell-based therapies, and angiogenesis approaches are together, trying to achieve, said Prof. Naoki Yamamoto, a co-researcher.
GNCorp, which spearheaded this accomplishment, has signed a MoU with MAP Healthcare, a subsidiary of SHBK International Holding Group, UAE and intends collaborating with like-minded organizations to validate Neu-REFIX in larger studies. Clinical research in Limb-Girdle Muscle Dystrophy (LGMD), multiple sclerosis and psoriasis are underway, to evaluate Neu-REFIX’s efficacy as a universal immune modulator.
*B-1,3-1,6 glucan is a listed food additive in MHLW, Japan; Not a drug or remedy to any illness. Research findings should not be construed as medical advice. Not GRAS, EFSA certified.
View source version on businesswire.com: https://www.businesswire.com/news/home/20230803923960/en/
Website: https://www.gncorporation.com/
Contact
GN Corporation Co Ltd
Samuel JK Abraham
info@gncorporation.com
This news is a press release provided by GN Corporation Co., Ltd..


